Innovation
Continuous innovations. One step ahead.
We anticipate tomorrow’s challenges to design smarter and forward-looking solutions in Dry Eye Management. Guided by innovations and purpose, one step ahead.
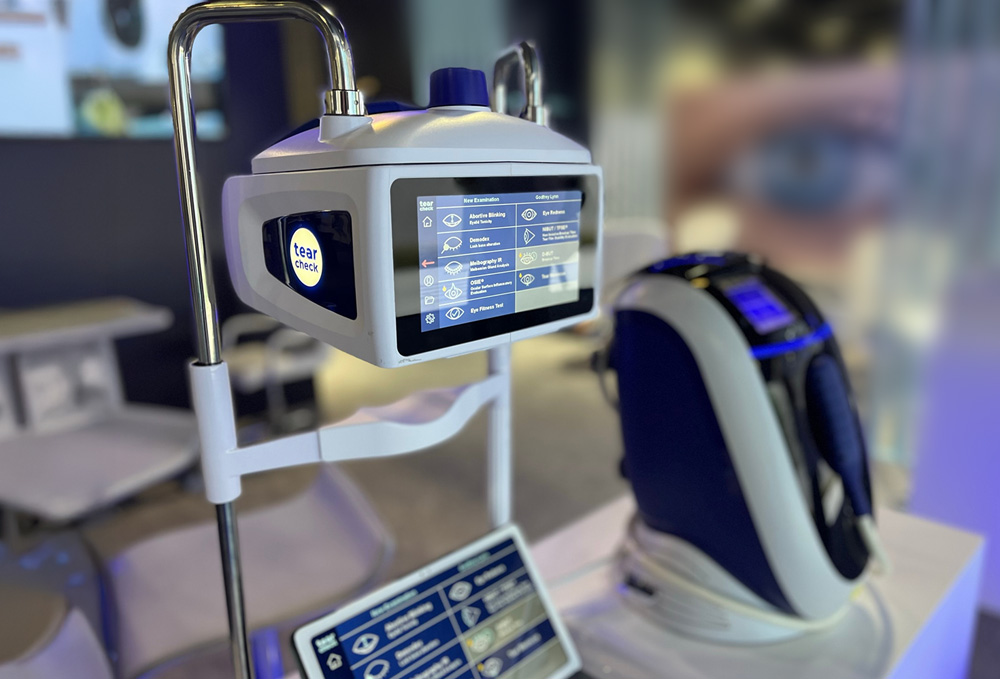
The patented IRPL® Technology
E-Eye is a medical device that has been specifically designed for treating dry eye syndrome due to MGD.
It generates Intense Regulated Pulsed Light by producing perfectly calibrated and homogeneously sequenced light pulses. The sculpted pulses are delivered under the shape of a train of pulses. The energy, spectrum and time period are precisely set to stimulate the Meibomian glands in order for them to return their normal function. E-Eye is the medical solution to treat and to prevent dry eyes.

Made in France. Available worldwide.

E-Swin’s manufacturing site is ISO 13485 and MDSAP certified, meeting EU, US, Canadian, Brazilian, and Australian standards.
All devices are CE-marked (MDR 2017/745) and registered in 60+ countries worldwide.
E-SWIN Certifications: ISO 13485 / MDSAP / CE (MDR 2017/745)
Product Registrations: EU (MDR 2017/745) / USA* (FDA) / Canada (HC) / Australia (TGA) / Brazil (Anvisa) / China (NMPA) / India (CDSCO) / KSA (SFDA)
*In the U.S., E-Eye is not FDA-cleared for Dry Eye Disease (DED) or Meibomian Gland Dysfunction (MGD).
Innovation driven by E-SWIN

Patented tearcheck® Line Mask made to create a new standard of ocular surface detection.

Patented regulated flash technology ensuring precise control of light emission in the target area.

Patented Air cooling system designed to prevent absorption of IR wavelengths that can occur with water cooling.
Voices from the experts

Eye & Laser Clinic Group
South Africa
“I’ve seen outstanding results in treating dry eye patients with E-Swin technology. The non-invasive, gentle treatment has significantly improved patient comfort. I highly recommend it to any colleague treating dry eye syndrome.”

Clinica Bine
Romania
“For the first time patients saw their results and the adherence to treatment was much better. On the other hand, the MGD treatment worked for them in a pleasant manner and with lasting results.”


Ophthalmic Consultants of Beirut
Lebanon
“Over the course of five years and 8000 treatments, I have consistently achieved excellent results for my patients. Its effectiveness as an additive to our dry eye clinic has been undeniable, significantly enhancing our treatment options and patient outcomes.”

Bowden Eye Associates
Florida, USA
“I have used other IPL devices, but the E-SWIN E-EYE IRPL is now my favorite IPL to use. I see as good or better results with the E-EYE upgraded technology with the need for fewer pules per patient treatment. (...) We couldn’t be happier with the E-EYE"

California Lasik & Eye
California, USA
“I have seen a tremendous impact of the E-Eye on ocular surface optimization in my patients, my family and myself. Great device! On top of that, the E-Swin team is incredibly supportive all around. Very happy customer!!”

Forsight Vision
Illinois, USA
Dr. Todd Cohan is a leading optometrist in Illinois specializing in vision therapy, pediatrics, and ocular disease management, known for integrating advanced technology into personalized patient care.

Augenzentrum Salzburg Süd
Austria
“We started using E-Eye in 2023 and have already achieved considerable success […] we added tearcheck to our equipment - a real game changer for our practice. We also particularly appreciate the extensive marketing materials such as flyers, videos and other supporting material that are made available to us.”

Oftalmocenter Clínica e Cirurgia
Brazil
“As a user of E>Eye, I am delighted. The satisfaction of my patients with lasting relief from Dry Eyes makes my work particularly fulfilling.”

ADVALIA - CARONES Vision
Italy
“E-Eye is the best device […]. I have been using IRPL treatment for evaporative dry eye disease with MGD for more than 8 years and my results are really impressive! Every time I thank God for having decided to try the instrument many years ago and to bring it in Italy!”

Clinica de Ojos Córdoba
Argentina
“When addressing dry eye issues, tearcheck® serves as a straightforward and invaluable tool, offering critical insights into the tear film and the condition of the ocular surface.”

Ophthalmologic Center Etoile
France
“I have been using E-Eye for about 10 years. I noticed a clear reduction in symptomatology in more than 85% of patients after 3 or 4 sessions and control at 3 months. The effectiveness of the treatment is about fifteen months. Patients are satisfied with the treatment.”
Eye7 Eye Hospitals
India
“I am using tearcheck® and E-Eye […]. The patients are really benefitted with the treatment therapy. The diagnosis report in tearcheck® is easy to understand by the patient’s and saves lot of precious time of consultants. I am more than happy using Tearchek and E-Eye to my OPD patients with very good results.”

Sie werden Sehen
Germany
“We currently carry out an average of ten screenings a day with tearcheck® and have noticed how impressed customers are with the clear and comprehensible presentation of the results. The feedback from customers is consistently positive - they are very satisfied with both the application and the results achieved. We particularly appreciate the excellent service and continuous support from the sales force.“

Optik Sutter
Austria
“We currently carry out around 15 screenings every day [with tearcheck®], resulting in 2-5 tearstim® application protocols per week. I am particularly impressed by the seamless integration of the devices into our customer management and network system. tearcheck® and tearstim® are now an indispensable part of our everyday work.”
Get in touch with our team to learn more

