












Innovative breakthrough solutions to manage dry eye.

Medical solution (Ophthalmology)

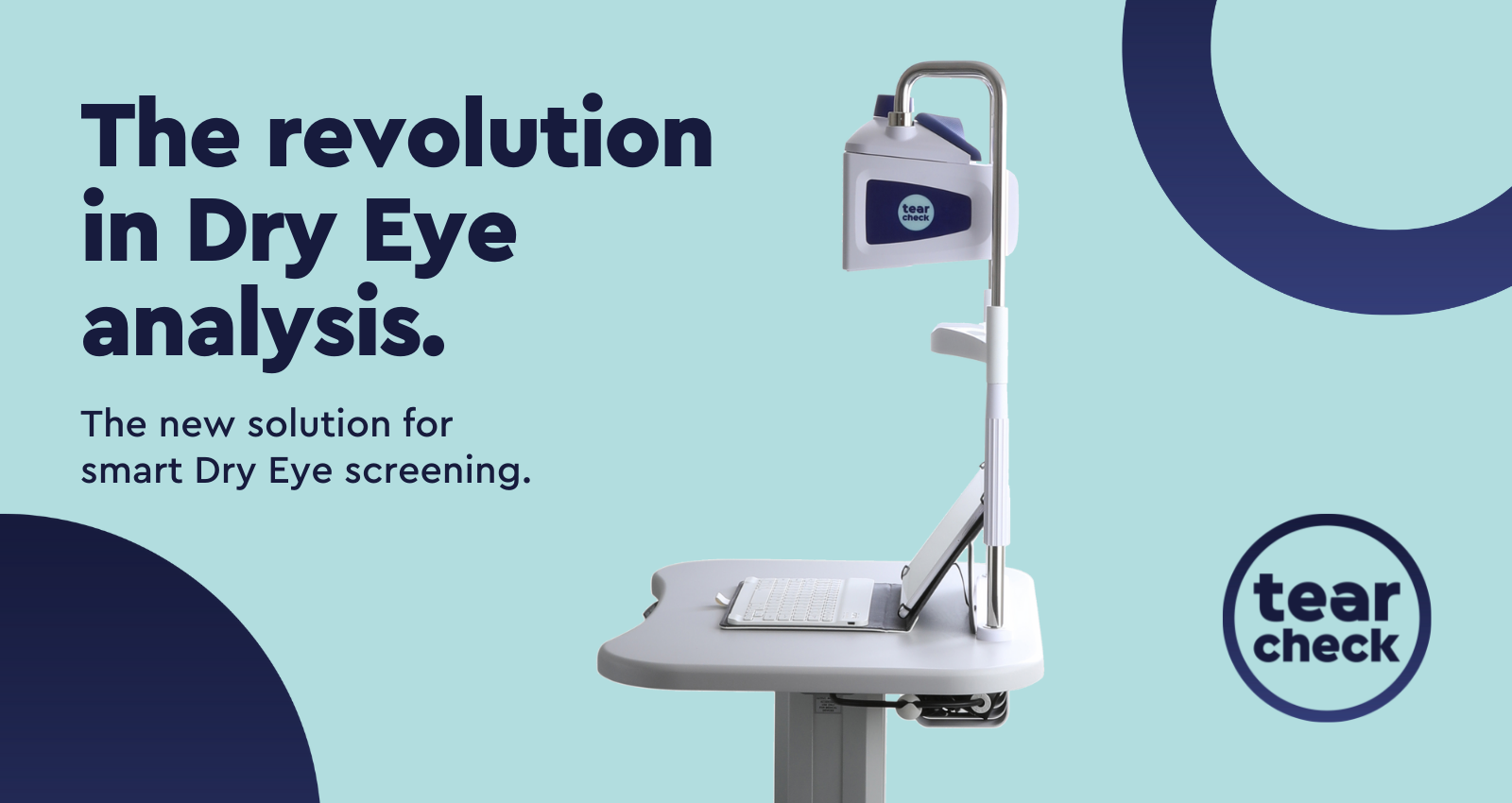
Optical solution
(Optometry/Opticians)

Medical solution for dry eye analysis
(Ophthalmology / Optometry / Opticians)




“I have seen a tremendous impact of the E-Eye
on ocular surface optimization in my patients,
my family and myself. Great device!
On top of that, the E-Swin team is incredibly
supportive all around. Very happy customer!!”
-
Dr. Bradley Barnett - California Lasik & Eye
“I have seen a tremendous impact of the E-Eye
on ocular surface optimization in my patients,
my family and myself. Great device!
On top of that, the E-Swin team is incredibly
supportive all around. Very happy customer!!”
-
Dr. Bradley Barnett - California Lasik & Eye
“I have seen a tremendous impact of the E-Eye
on ocular surface optimization in my patients,
my family and myself. Great device!
On top of that, the E-Swin team is incredibly
supportive all around. Very happy customer!!”
-
Dr. Bradley Barnett - California Lasik & Eye
"I have used other IPL devices, but the E-SWIN E-EYE IRPL is now my favorite IPL to use. I see as good or better results with the E-EYE upgraded technology with the need for fewer pules per patient treatment. The patients who have undergone other IPL treatments with older platforms tell me that the E-EYE is much more tolerable and that they are very pleased with the results in comparison. The training and implementation with the E-SWIN team was simple and easy.
We couldn’t be happier with the E-EYE"
-
Dr. Jerry Robben – Bowden Eye & Associates
"I have used other IPL devices, but the E-SWIN E-EYE IRPL is now my favorite IPL to use. I see as good or better results with the E-EYE upgraded technology with the need for fewer pules per patient treatment. The patients who have undergone other IPL treatments with older platforms tell me that the E-EYE is much more tolerable and that they are very pleased with the results in comparison. The training and implementation with the E-SWIN team was simple and easy. We couldn’t be happier with the E-EYE"
-
Dr. Jerry Robben – Bowden Eye & Associates
"I have used other IPL devices, but the E-SWIN E-EYE IRPL is now my favorite IPL to use. I see as good or better results with the E-EYE upgraded technology with the need for fewer pules per patient treatment. The patients who have undergone other IPL treatments with older platforms tell me that the E-EYE is much more tolerable and that they are very pleased with the results in comparison. The training and implementation with the E-SWIN team was simple and easy. We couldn’t be happier with the E-EYE"
-
Dr. Jerry Robben – Bowden Eye & Associates
Satisfaction Survey
Please take a moment to share your thoughts in the following
Satisfaction Survey. Your insights are helping us improve
your customer journey. We appreciate your
time and continued support.
Join our experts on connect® Round Tables
Insights

2024 BARCELONA
E-Swin Charity
We are proud to introduce our new Charity program, E-Swin Charity dedicated to creating a healthier, more sustainable future for communities around the world...
At the APAO/AIOS Congress 2025 in New Delhi, ESW vision and Appasamy Associates officially announced their strategic partnership.

We are looking forward to welcoming you at one of the biggest Ophthalmology events of the year together with DEM Services. Visit us at our booth SP-218 & SP-233!

2024 BARCELONA
September
Join us at ESCRS 2024 in Barcelona for an exclusive look at cutting-edge solutions in ophthalmology. Don't miss the opportunity to explore the future of eye care with us!

2024 MANNHEIM
June
Successful conference, where innovative Dry Eye Management technologies garnered significant interest and enjoyed insightful discussions.. More insights...

